
Cutting-edge immunological biomarker and its role in managing infections and immune responses.
BioVendor Group presents MxA protein, a unique biomarker of viral activity. Differentiation between viral and bacterial infections for effective antibiotic treatment – on three different platforms.
Why to Avoid Overuse of Antibiotics?
Antimicrobial resistance is a global public health challenge, which has been accelerated by the overuse of antibiotics.
Increased antimicrobial resistance leads to severe infections, complications, longer hospital stays, and increased mortality. This issue is particularly prevalent in primary care, where most infections are viral. General practitioners issue about 90% of all antibiotic prescriptions, primarily for respiratory tract infections. Overprescribing antibiotics not only contributes to resistance but also increases the risk of adverse effects and frequent patient re-attendance.
Antibiotic overuse issue is not limited to respiratory tract infections in adults. It also significantly impacts vulnerable populations, such as newborns. Effective interventions to reduce antibiotic overuse include improved laboratory tests or reliable rapid point-of-care tests to reduce diagnostic uncertainty. Overprescribing antibiotics not only contributes to resistance but also increases the risk of adverse effects and frequent patient re-attendance.
In newborns, especially preterm ones, overuse impairs immune system maturation, making them more susceptible to infections and other immune-related conditions. Antibiotics can affect the development and function of T and B cells, crucial for adaptive immunity. Acute respiratory infections (ARIs) account for 41% of all outpatient antibiotics, with about half being medically unnecessary. Moreover, antibiotic resistance is estimated to cost double compared to antibiotic treatment.
Simple, inexpensive tests such as MxA assay that can guide antibiotic prescription in the outpatient setting have been identified as a successful strategy to reduce inappropriate prescription of antibiotics. MxA assay can rule out viral infection with up to 95% sensitivity and 94% specificity. As such the assay allows to identify appropriate candidates for antibiotics and reducing errors.
Economic benefits of the MxA test include less outpatient visits, emergency visits, hospitalizations, and reduced costs related to antibiotic resistance.
MxA: A Key Player in the Immune Response to Viral Infections
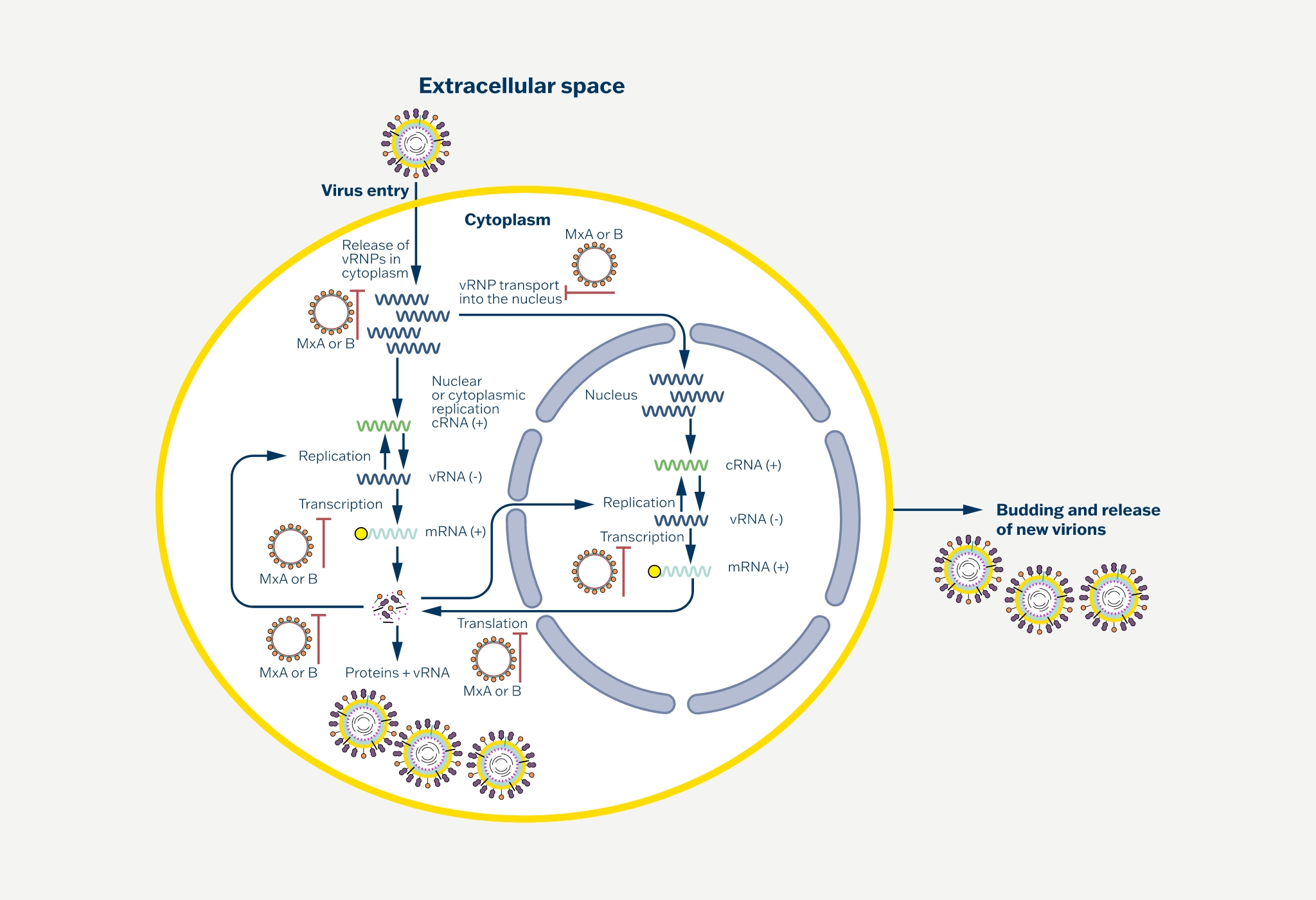
Human MxA protein (Myxovirus resistance protein 1), encoded by the MX1 gene, is a 76-kDa protein composed of 662 amino acid residues and is a member of the dynamin superfamily of large GTPases. The MxA protein plays a crucial role in antiviral defense within cells, providing protection against a broad range of viruses, including influenza, parainfluenza, measles, coxsackie, hepatitis B, and Thogoto viruses.
The viruses are inhibited by MxA protein at an early stage in their life cycle, soon after host cell entry and before genome amplification. The human MxA protein is accumulated in the cytoplasm and endoplasmic reticulum. The membrane compartment of endoplasmatic reticulum seems to provide an interaction platform that facilitates viral target recognition.
MxA appears to detect viral infection by sensing and trapping nucleocapsid structures, and becoming the viral components unavailable for the generation of new virus particles. The expression of viral MxA protein is induced exclusively and, in a dose-dependent manner, by IFN-alpha and IFN-beta, but not by IFN-gamma, IL-1, TNF-alpha or other cytokines.
New Insights into Clinical Diagnostics
MxA protein with its low basal concentration and long half-life, offers advantages as a marker for viral infection. Clinical studies have reported on MxA protein in peripheral blood mononuclear cells as a marker distinguishing viral from bacterial disease, and as a reliable marker for type I IFN bioavailability during IFN treatment in patients with multiple sclerosis (MS) according to the recommendation from EMA (European Medicine Agency).
Who Can Benefit from MxA Assay?
The MxA assay can benefit a range of healthcare providers and patients. It is useful for patients with upper respiratory tract infections, blood biobanks for donor screening, and hospitals for monitoring organ transplant recipients or conducting preoperative and postoperative investigations.
Private medical care centers, particularly those with travel and tropical medicine departments, can use the assay to screen for blood-borne tropical viral fevers such as dengue, malaria, and yellow fever. Additionally, the test can be helpful to evaluate disease activity of Sjögren's syndrome (to stratify patients by IFN positivity) and multiple sclerosis (to monitor treatment efficacy in patients receiving interferon beta) in specialized healthcare centers.
From research to IVD – three platforms offering a comprehensive solution
We have been offering Human MxA ELISA for more than 10 years for research purposes, and many reserch studies were published with the assay.
Currently, BioVendor Group is launching a new point-of-care lateral test with CE IVD certification. A brand new CLIA assay developed by BioVendor Group laboratories on the KleeYa® platform is expected soon. This test is designed for routine laboratory analysis with automated operation. Overall, we offer the appropriate solution to suit the type of user, including research laboratories, outpatient clinics, hospital laboratories and global supply chain laboratories.
Discover cutting-edge solutions for MxA diagnostics
Choose the test that best fits your needs:
ELISA: MxA Protein Human ELISA
POCT: Bi-VirTest for Human MxA
CLIA: MxA CLIA Kit
